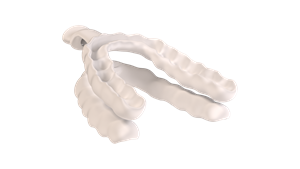
Nylon flexTAP®: First FDA cleared 3D-Printed Midline Oral Appliance for Sleep Apnea
DALLAS, Sept. 17, 2025 (GLOBE NEWSWIRE) -- Airway Management proudly announces a historic milestone with the FDA clearance of Nylon flexTAP®, the world’s first digitally printed single-point midline oral appliance device for sleep apnea. Launched today, this revolutionary device redefines patient care by combining cutting-edge patented Vertex Technology® with unparalleled comfort and efficacy, now FDA cleared for mild to moderate obstructive sleep apnea (OSA).

Key Features and Benefits
- Digitally Printed Medical-Grade Nylon: Utilizes advanced digital printing for a precise, custom-fit design that prioritizes patient comfort.
- Patented Vertex Technology®: Enables dual-axis (vertical and horizontal) movement to maximize airway space, reducing the need for excessive protrusion—ideal for patients with larger tongues or narrow airways.
- 17mm Range of Advancement: Features precise 1/3 mm increment adjustments for optimal mandibular protrusion, improving airway patency.
- Ultra-Thin Custom TAP Trays: Offers maximum comfort without compromising treatment effectiveness. The Thinnest Custom TAP yet.
- BPA-Free and Metal-Free: Crafted with biocompatible materials for enhanced safety.
- No Bite Registration Required: Simplifies the fitting process, saving time for clinicians and patients.
- Mouth Shield and AM Aligner® Included: The only oral appliance to include a nasal breathing accessory improving efficacy. The AM Aligner is a morning exercise tool mitigating common side effects associated with oral appliance therapy (OAT).
- 4-Year Warranty: Ensures long-term reliability and confidence.
“We are thrilled to introduce the Nylon flexTAP, a game-changer in oral appliance therapy,” said Charles Collins, CEO of Airway Management. “Feedback from key opinion leaders in our TAP Sleep Care system highlights its superior effectiveness and patient comfort. Peer-reviewed studies also confirm that our patented mouth shield, which promotes nasal breathing, significantly improves treatment outcomes. This innovation underscores our commitment to advancing sleep health.”
A New Standard in Sleep Apnea Treatment
Since 2015, the American Academy of Sleep Medicine (AASM) and American Academy of Dental Sleep Medicine (AADSM) have recommended oral appliance therapy for mild to moderate sleep apnea. The Nylon flexTAP sets a new benchmark by leveraging digital printing technology to create the first FDA-approved single-point midline device, eliminating the need for bite registration while maintaining precision.
Manufactured in the U.S.A., the device is now available through Airway Labs, with PDAC coverage (E0486) pending.
To learn more or get started with Nylon flexTAP, contact Airway Management today.
About Airway Management, Inc.
Airway Management, Inc., the innovator behind TAP Sleep Care, is a leader in advanced oral appliances, supported by over 50 independent peer-reviewed studies—more than any other manufacturer—demonstrating proven efficacy in treating sleep-disordered breathing. Our TAP devices, including myTAP, flexTAP, and hybrid solutions like custom TAP PAP, provide comfortable, minimally invasive alternatives to CPAP, empowering patients to achieve restful sleep and improved quality of life. Committed to excellence, we support clinicians, healthcare professionals, and laboratory technicians with high-quality, customizable therapies that address snoring and obstructive sleep apnea, helping patients wake up rested, refreshed, and recharged.
Airway Management Contact
Press Inquiries: Kelly Grant, Marketing, kgrant@amisleep.com
Toll free, 866-264-7667
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/1969c53f-b20c-4261-802b-879ca267a022
https://www.globenewswire.com/NewsRoom/AttachmentNg/43c838e6-f185-4b95-9911-75ef603cf6d3
https://www.globenewswire.com/NewsRoom/AttachmentNg/a661b496-5168-4f39-a086-16a5b0febc21
https://www.globenewswire.com/NewsRoom/AttachmentNg/2183554f-a2b4-4a0f-941f-4136a66089ce
A video accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/881bbb2d-75b9-47c5-a8c4-28c8469b83f1

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.